A Biased View of FDA alerts providers to COVID-19 rapid test recall - AHA News


State health department pauses rapid COVID testing
About iHealth COVID-19 Rapid Antigen Test - The First Aid Gear Shop

Makers must examine the effect of new variants of public health issue on their test, taking into account efficiency and labelling, and include this assessment in their application. If it's included in the sent in-silico and/or wet screening, this need to be mentioned clearly. Another Point of View should show how they plan to alleviate any new threats, including timelines for resolving these threats.
Labels need to include a declaration that includes the following limitation or one that communicates the very same significance: "The performance of the device has not been evaluated on specimens from people who have been contaminated with emerging versions of SARS-Co, V-2 of public health concern." Manufacturers that send proof of their device's efficiency in specimens from individuals contaminated with emerging variants might be able to have this requirement changed.
Review the submission requirements for antigen-based devices supplied on this page. Prepare your submission package. Each submission needs to consist of enough information, including appropriate test data and gadget labelling, so that Health Canada can authorize the device. Send your application to the Medical Gadgets Directorate at . For information about the licensing or permission of medical devices in Canada, please contact the Medical Devices Directorate at meddevices-instrumentsmed@hc-sc.
The Best Strategy To Use For 8 Best At-Home Rapid Antigen COVID-19 Tests To Buy Online
Nu qu v c cc triu chng ca COVID19 hoc c th b phi nhim, iu quan trng l cn c xt nghim ngay. Xt nghim sm gip ngn nga COVID19 ly lan cho bn b, gia nh, v cng ng. Qu v c th thc hin iu ny ti nh, s dng b dng c t xt nghim n gin v d s dng, v nhn kt qu ch sau 15-30 pht.
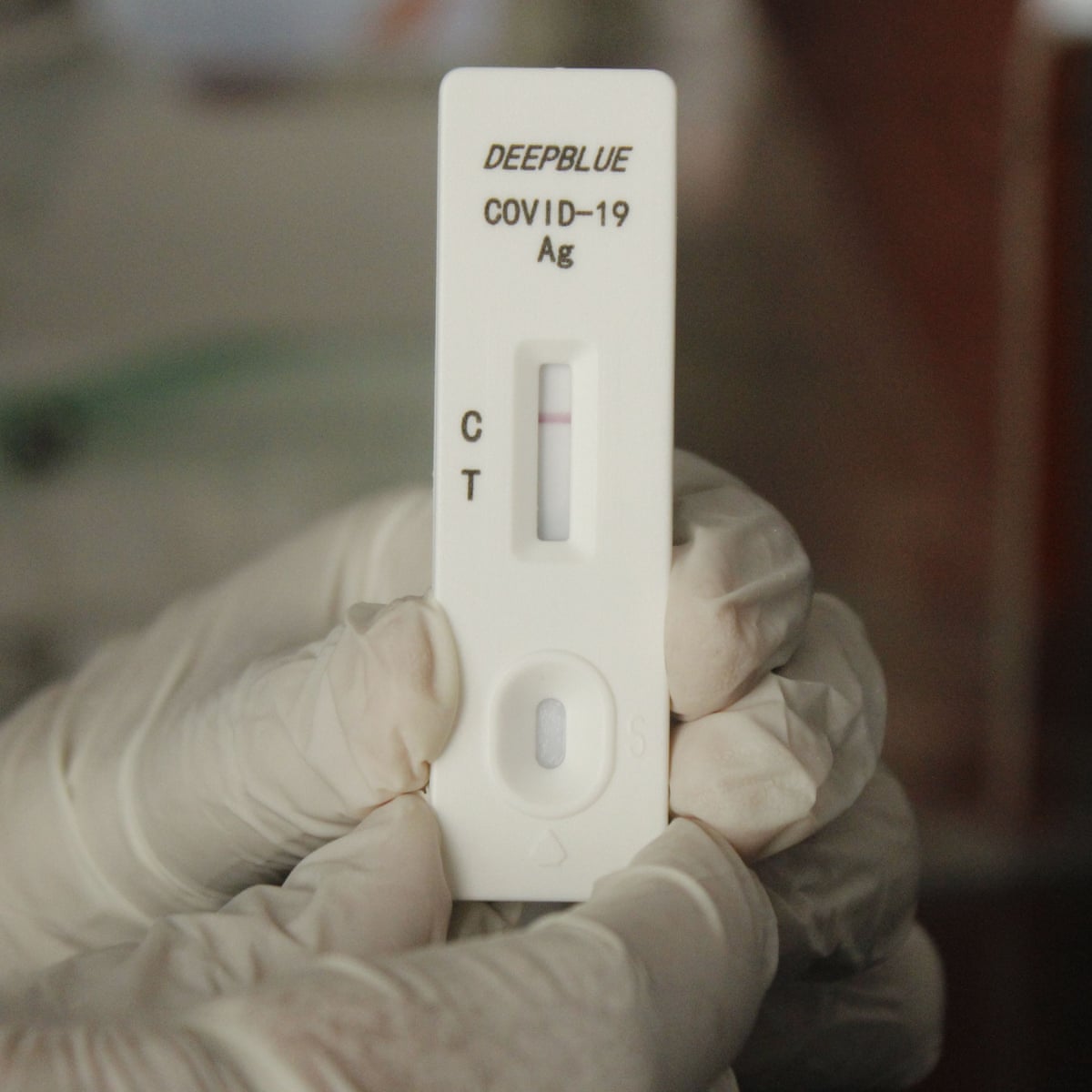
Why home COVID tests remain costly and hard to get in the U.S: Shots - Health News : NPR
be/yz, Aqnkc, Mw, Q8 external link .
We motivate you to document your favorable fast test lead to the event you may require to share the results with somebody. To make this easier, finish the following kind and share it in addition to a photo of the fast test result with whomever you require to. This form is not valid evidence of a recent test for the functions of the Restrictions Exemption Program.
